Follow With Interest
More
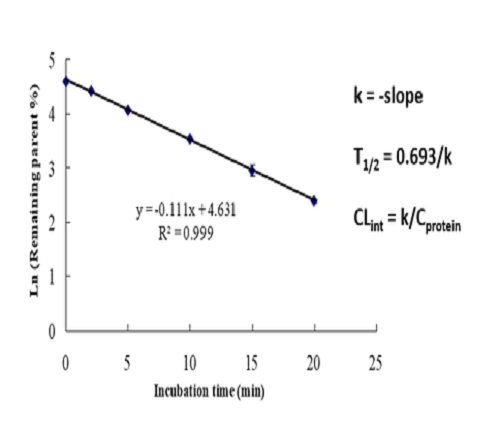
Metabolic stability test is used to determine the elimination half-life and in vitro clearance rate of the test substance in the incubation system, so as to understand the metabolism of the test substance, and provide reference for subsequent metabolite identification, metabolic pathway identification or selection of pharmacokinetic and toxicological animal species.
Incubation system | Subcellular components: microsomes, S9 or homogenate of liver, intestine, lung or kidney tissues Cell components: liver cells, human epithelial cells HACAT Gene recombinase system Other tissues, cells or subcellular components: available upon request (such as plasma, artificial gastric fluid, artificial intestinal fluid, etc.) |
Species | CD1 mice, SD rats, beagle dogs, cynomolgus monkeys, miniature pigs, New Zealand rabbits, human (other species or strains can be provided upon request) |
Test concentration of the test substance | To Be Determined (different test concentrations can be provided upon request) |
Protein concentration of the incubation system | To Be Determined (different protein concentrations can be provided upon request) |
Incubation time | To Be Determined (can be provided according to different requirements) |
Coenzyme factors | Subcellular components: NADPH and/or UDPGA (other coenzyme factors can be provided upon request) |
Analytical method | LC-MS/MS |
Data presentation | Elimination half-life (T1/2) In vitro clearance rate (CLint) Extraction rate (ER) or other parameters |